This article focuses on one of the most critical types of training programs – compliance training and how you can use it to achieve regulatory adherence – if your organization operates in the pharmaceutical domain. We will also explore how to design effective compliance training modules to address the unique needs of pharmaceutical companies. Finally, we’ll discuss the common pitfalls in pharmaceutical compliance and how the right training can address and mitigate these challenges.
In the pharmaceutical landscape, the stakes are undeniably high. Ensuring regulatory adherence isn’t just a box to tick; it’s a matter of public health, patient safety, and industry integrity. Imagine the weight of responsibility on your shoulders as a pharmaceutical professional. It’s not merely about manufacturing pills or developing breakthrough drugs; it’s about doing so within a tightly woven web of regulations and guidelines.
Consider this: In recent years, the pharmaceutical industry has seen a surge in regulatory scrutiny. The U.S. FDA conducted over 2,500 inspections in 2023 alone, resulting in thousands of observations and warning letters.
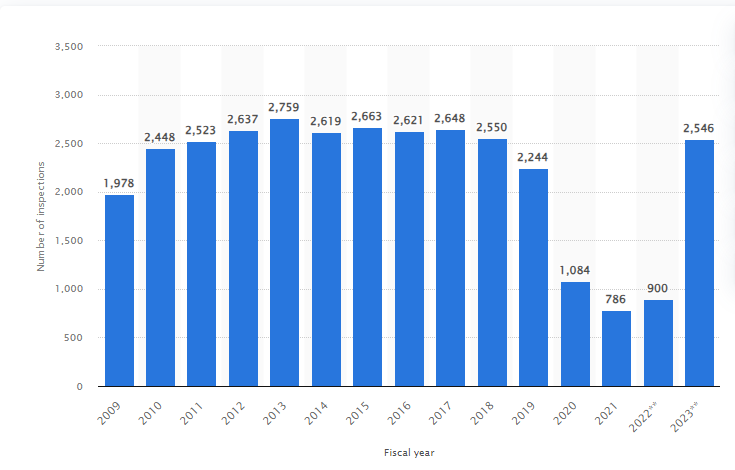
Globally, regulatory authorities are intensifying their focus on pharmaceutical companies, demanding strict compliance with standards that evolve with scientific advancements and emerging risks.
Now, enter compliance training—a formidable ally in your quest for regulatory adherence. It’s not just another corporate obligation; it’s your compass in the complex regulatory landscape. In this article, we’ll navigate this crucial terrain together, exploring how compliance training serves as the lighthouse guiding your pharmaceutical ship safely through turbulent regulatory waters.
Table of Contents
What Are the Standard Pharmaceutical Regulations?
As you step into the intricate world of pharmaceuticals, it’s essential to grasp the complexity of regulatory landscapes that govern this industry. Picture it as a multifaceted puzzle, where each piece represents a rule, guideline, or requirement crucial to your operations.
Regulatory Bodies
You’ll encounter a multitude of regulatory bodies, but some of the heavyweights include the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and their counterparts worldwide. These entities are the architects of the rules that ensure the safety, quality, and efficacy of pharmaceutical products.
Compliance and Consequences
Now, here’s where the stakes get high. Non-compliance isn’t a mere oversight; it can lead to grave consequences. Think hefty fines, product recalls, damaged reputations, and even legal actions. For instance, in 2020, a major pharmaceutical company faced a staggering $678 million fine for violations of anti-kickback laws.
So, as you navigate this intricate regulatory terrain, remember that understanding pharmaceutical regulations isn’t a choice—it’s a necessity. It’s your compass to steer clear of treacherous compliance waters and safeguard both your organization’s reputation and, more importantly, the health and well-being of patients relying on your products.
Ad: PlayAblo’s Enterprise-Grade Micro-Learning platform is built for millennial learners. Micro-Learning, assessments, and gamification features ensure learning outcome measurement and sustained engagement.
Find out more and request a custom demo!
What Is the Importance of Compliance Training?
Now, let’s dive into a crucial aspect of your journey toward regulatory adherence in the pharmaceutical world—the pivotal role of compliance training. This isn’t your run-of-the-mill training; it’s the shield that guards your organization against regulatory violations and the legal quagmire that may follow.
- Guardians of Compliance: Imagine compliance training as your organization’s guardians, diligently patrolling the boundaries of regulatory compliance. Their primary mission? Educate every member of your pharmaceutical team about the rules, guidelines, and ethical standards governing your industry.
- The Power of Prevention: Here’s where the significance becomes crystal clear. Effective compliance training is your proactive defense against regulatory violations. It equips your workforce with the knowledge and understanding needed to spot potential pitfalls and navigate the regulatory maze safely. Statistics reveal that companies with robust compliance training programs can save up to $1.03 million on average.
- Legal Lifesaver: But it doesn’t stop there. Compliance training can be your legal lifesaver. In a world where pharmaceutical companies face an increasing number of lawsuits, having a well-trained workforce can be the difference between a costly legal battle and a smoothly resolved issue.
How to Design the Right Types of Training Programs for Pharma Compliance?

Now, let’s embark on the journey of crafting an effective compliance training program tailored to the intricate demands of pharmaceutical rules. This isn’t a one-size-fits-all endeavor; it’s about creating a finely-tuned instrument that addresses the specific needs and challenges of your industry.
- Understanding the Pharmaceutical Landscape: First and foremost, you’ll need to assess the unique landscape of pharmaceutical compliance. It’s unlike any other industry, with regulations that can change swiftly and stakes that are remarkably high. By understanding these dynamics, you can better tailor your training efforts.
- Identifying Key Stakeholders: Compliance training isn’t a solo mission. It involves a cast of crucial characters, from subject matter experts to legal advisors. Identifying these key stakeholders and involving them in your training program ensures a comprehensive approach.
- Customization is Key: Cookie-cutter training won’t cut it in pharmaceutical compliance. You’ll need to craft a customized curriculum that aligns with your organization’s specific activities and the regulatory requirements you face. This tailored approach ensures that your team receives the precise knowledge they need.
- Setting Clear Objectives: In the world of compliance training, ambiguity is your enemy. Establishing clear learning objectives is essential. What do you want your team to know, understand, and do as a result of the training? Clear objectives not only guide the curriculum but also allow you to measure outcomes effectively.
- Measuring Success: Speaking of measurement, ensuring that your training program produces measurable outcomes is vital. It’s not just about ticking a box; it’s about assessing whether your team is better equipped to handle compliance challenges. Metrics like reduced incidents of non-compliance and improved audit outcomes provide tangible proof of success.
How to Improve Compliance Training With Technology?

Welcome to the digital frontier of compliance training—a realm where technology becomes your ally in navigating the complexities of pharmaceutical regulations:
- E-Learning Revolution: Picture a world where training isn’t confined to a stuffy classroom but extends into the digital realm. E-learning offers flexibility, scalability, and accessibility like never before. It allows your team to access training materials anytime, anywhere, making it an ideal fit for the dynamic nature of pharmaceutical compliance.
- Real-World Success Stories: To truly appreciate the impact of technology-driven training, showcase real use cases. These success stories illustrate how pharmaceutical companies have harnessed the power of e-learning and digital tools to enhance compliance training, reduce incidents of non-compliance, and streamline the learning process.
- Choosing the Right Tools: With technology comes choices, and selecting the right compliance training software or platform is crucial. You can pick from several online training tools like microlearning, mobile learning, gamification, and learning management systems. Factors like user-friendliness, scalability, and reporting capabilities should guide your choice.
Ad: PlayAblo’s Enterprise-Grade Micro-Learning platform is built for millennial learners. Micro-Learning, assessments, and gamification features ensure learning outcome measurement and sustained engagement.
Find out more and request a custom demo!
How to Engage Learners in Compliance Training?
Now, let’s delve into a critical aspect of compliance training—engaging your learners effectively. It’s not just about delivering information; it’s about creating an interactive and immersive learning experience that resonates with your pharmaceutical team.
- Interactive Strategies: Compliance training doesn’t have to be a monotonous checklist. We’ll explore innovative strategies that make learning not only informative but engaging. From gamification to collaborative exercises, discover how to turn compliance training into an active, participatory experience.
- Real-Life Connection: The power of real-life scenarios, case studies, and simulations cannot be overstated. We’ll show you how to incorporate these elements into your training, allowing your team to connect theory to practice. By confronting real-world challenges in a safe learning environment, they’ll be better prepared to handle them in the field.
Continuous Monitoring and Improvement in Pharmaceutical Training

In the pharmaceutical industry, compliance training isn’t a one-and-done endeavor; it’s an ongoing process. To navigate the complex terrain of regulatory adherence effectively, continuous monitoring and improvement are essential.
- Assessing Effectiveness: Compliance training effectiveness isn’t static. It needs regular assessment to ensure it remains relevant. Consistently measure the program’s impact, assess knowledge retention, and identify areas that need refinement. This ongoing evaluation not only ensures compliance but also equips your team to meet evolving regulatory challenges.
- The Power of Feedback: Learners and stakeholders provide valuable insights. Establish a robust feedback loop to gather their perspectives on training efficacy. Their input helps fine-tune your training approach, ensuring it aligns with real-world needs.
- Adaptation: The pharmaceutical landscape is ever-changing. Regulations shift, risks evolve, and technology advances. Adapt your training programs accordingly to stay compliant and competitive. This proactive approach prepares your team for current and future compliance challenges.
Case Studies
Let’s explore two hypothetical case studies of pharmaceutical companies that have excelled in regulatory adherence through their comprehensive compliance training programs.
Case Study 1: PharmaCo
| Company Name | PharmaCo |
|---|---|
| Challenge | PharmaCo faced increasing regulatory scrutiny and the need to ensure that all employees, from R&D to manufacturing, were well-versed in compliance requirements. |
| Solution | They implemented a tailored compliance training program that included e-learning modules, real-life case studies, and regular assessments. |
| Benefits | – Reduced instances of non-compliance by 40% |
Case Study 2: BioMed Solutions
| Company Name | BioMed Solutions |
|---|---|
| Challenge | BioMed Solutions aimed to align its compliance practices with the latest global regulations, spanning multiple markets. |
| Solution | They adopted a technology-driven approach, utilizing a Learning Management System (LMS) and mobile-accessible training modules. |
| Benefits | – Achieved 100% compliance across international markets |
Regulatory Challenges in Pharmaceuticals

The pharmaceutical industry operates in one of the most tightly regulated landscapes globally. Navigating this complex terrain comes with a set of unique challenges:
Common Roadblocks and Pitfalls
- Evolving Regulations: Pharmaceutical regulations are constantly evolving. Keeping up with these changes, understanding their implications, and ensuring compliance can be a daunting task.
- Global Market Expansion: As pharmaceutical companies expand into international markets, they must grapple with diverse and often conflicting regulatory requirements.
- Stringent Quality Standards: Maintaining high-quality standards is non-negotiable in pharmaceuticals. Any deviation can lead to severe consequences, including product recalls.
- Data Security and Privacy: With the increasing reliance on digital technologies, safeguarding sensitive patient data and complying with data protection regulations is a significant challenge.
- Employee Turnover: High turnover rates in the industry mean that continuous training and knowledge transfer are essential to maintain compliance.
Addressing Challenges with Compliance Training
Compliance training emerges as a powerful solution to tackle these challenges:
- Regulatory Updates: Regular training programs can keep employees informed about evolving regulations and their implications for daily operations.
- Global Understanding: Tailored compliance training modules can provide insights into international regulations, ensuring that employees are well-prepared for market expansion.
- Quality Assurance: Training programs emphasize the importance of stringent quality standards, reducing the likelihood of quality-related compliance issues.
- Data Compliance: Training can educate employees on data security protocols and privacy regulations, minimizing the risk of data breaches.
- Continuous Learning: Compliance training facilitates ongoing learning and knowledge transfer, ensuring that expertise remains within the organization despite employee turnover.
Regulatory Adherence Beyond Training: A Holistic Approach
Compliance training is a cornerstone of regulatory adherence in the pharmaceutical industry, but it’s just one piece of the puzzle. To truly excel in the realm of compliance, a holistic approach is essential.
A Culture of Compliance
- Leadership Engagement: Compliance starts at the top. Leadership commitment to compliance sets the tone for the entire organization. Leaders must not only endorse but actively participate in compliance initiatives.
- Cultural Integration: Compliance isn’t a separate entity; it should be woven into the fabric of your organization’s culture. Employees should understand that compliance is everyone’s responsibility.
Accountability and Transparency
- Clear Accountability: Establish clear roles and responsibilities for compliance within your organization. Ensure that individuals are held accountable for their compliance-related duties.
- Transparency: Open communication channels for reporting compliance concerns or potential violations. Encourage a culture where employees feel safe reporting issues without fear of retaliation.
Integration into Operations
- Strategic Decision-Making: Compliance should be a key consideration in strategic decision-making processes. Assess how decisions impact compliance and take proactive steps to address any potential issues.
- Daily Operations: Ensure that compliance isn’t an afterthought but an integral part of daily operations. From manufacturing practices to data security protocols, compliance should be ingrained in every aspect of your organization.
- Regular Auditing: Implement regular internal audits to assess compliance levels. Identify areas that may need attention and make necessary improvements.
Wrapping It Up
In the pharmaceutical industry, regulatory adherence isn’t confined to a training room; it’s a philosophy that must permeate every facet of your organization. It requires leadership commitment, cultural integration, accountability, and a proactive approach to daily operations and decision-making.
By embracing this holistic approach, pharmaceutical companies can not only meet regulatory requirements but also build a reputation as industry leaders committed to ethics and excellence.
Ad: PlayAblo’s Enterprise-Grade Micro-Learning platform is built for millennial learners. Micro-Learning, assessments, and gamification features ensure learning outcome measurement and sustained engagement.
Find out more and request a custom demo!







Comments are closed, but trackbacks and pingbacks are open.